The Mitochondria: Key to Health and Longevity
ARTICLE SUMMARY
• Mitochondria generate 90 percent of the body’s energy, powering the cells to perform vital functions.
• Mitochondria burn two main fuels: glucose and ketones. Because the standard American diet has given rise to a widespread sugar addiction, most people tend to burn glucose.
• When the mitochondria are healthy, a person is generally healthy.
• Mitochondrial deficiency can give rise to symptoms in virtually any organ or tissue.
• Many factors—chemicals, foods, modern lifestyle habits and electromagnetic fields—have been implicated as causes of mitochondrial dysfunction.
• In cancer, the mitochondria change first—before any gene coding changes—which undermines the “gene theory” premise of conventional cancer explanations.
• Dysfunctional mitochondria can trigger a series of cellular processes that result in insulin resistance (diabetes).
• Feeding and nourishing mitochondria with wholesome ketones—which do not require insulin—can help restore mitochondrial health and relieve brain starvation in individuals with cognitive impairment.
• AMPK is an enzyme responsible for multiple metabolic functions. AMPK and mitochondrial imbalances can initiate a cascade of inflammatory changes related to disorders such as obesity and diabetes.
• As key elements for cellular energy production, AMPK and mitochondria require nutrient and lifestyle changes when imbalanced.
• Supporting mitochondrial health can help control the aging process as well improve overall health.
For most of my professional life as an alternative family physician, I’ve understood that the frontier in medicine and health is a place where diseases without cures remain unsolved mysteries—awaiting the breakthroughs of discovery, connection, coincidence and evidence that will unravel and explain them. It is impossible to overstate the importance of unravelling these mysteries. Unfortunately, most family doctors and internists are unlikely to recognize or have prior knowledge about these troubling conditions and thus cannot guide their patients to proper medical treatment.
Sir Arthur Conan Doyle’s creation of one of the best-known fictional detectives, the iconic Sherlock Holmes, introduced a logical method for solving mysteries: “Once you eliminate the impossible, whatever remains, however improbable, must be the truth.” Ten years ago, I heard about a strange health condition—mitochondrial dysfunction—with documented links to other conditions such as autism and attention-deficit/hyperactivity disorder (ADHD), which may result from severe and damaging changes within the cells. Inspired by Doyle to retain my suspicions, my story begins with whatever remains, however improbable. Applying an understanding of mitochondria, I suspected the origins of and pursued the missing but probable explanation for this widespread health and medical problem.
WHAT ARE MITOCHONDRIA?
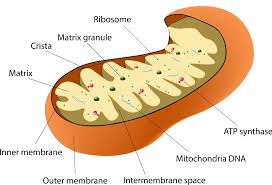
A brief course in cell biology is crucial to understanding the breakdown that leads to illness. Just as the body has vital organs—such as the heart, stomach and liver—the cell has similar critically functioning components. In the cell, these parts are called “organelles” and include the nucleus, Golgi complex, centrioles and mitochondria. Not confined to humans, mitochondria exist in all forms of life. Having unique DNA similar to the DNA of bacteria, mitochondria are actually a kind of parasite that lives in all organisms. By scientific estimate, eight to two thousand mitochondrial hitchhikers live in every cell of the human body.
The essential role of the mitochondria is to produce energy. Mitochondria generate 90 percent of the body’s energy and power the cells to perform vital functions that include breathing, thinking, talking and walking. How does this work? Inside the mitochondria, carbohydrates, fats and proteins are metabolized by utilizing oxygen to produce energy, which converts ADP (adenosine diphosphate) to the energy-packed ATP (adenosine triphosphate). Called the Krebs’s cycle, this process is a marvel of cellular biology, producing thirty-eight ATPs per molecule of glucose burned.
When the mitochondria are healthy, a person is generally fit and healthy. Moreover, under the influence of a positive cascade of effects, the more mitochondria that individuals have, the healthier they are, which increases their ability to create energy. Children brimming with energy, for instance, are loaded with mitochondria, whereas the less energetic elderly have fewer mitochondria at the cellular level.1 Recognizing that the mitochondria are essential to human health also leads to another conclusion, namely that health and disease processes are interrelated. According to a researcher writing in the Journal of Inherited Metabolic Disease, mitochondrial deficiency “can theoretically give rise to any symptom, in any organ or tissue, at any age, and with any mode of inheritance.”2
MITOCHONDRIA IN THE NEWS
Since the discovery of genes in 1954, a “gene theory” has become the most likely explanation for cancer, with researchers positing that humans develop cancer as a result of genetic changes in the cell. According to this theory, when the immune system does not identify or destroy a deformed cell, the altered cell continues to multiply, becoming a tumor, and the person eventually is diagnosed with cancer.
In 2003, the Human Genome Project completed its mapping of the entire human gene sequence, a thirteen-year undertaking that required the collaboration of twenty universities in seven countries. Scientists are now working on a new project—mapping the cancer genome to discover treatments—a task one thousand times greater than the original mapping of the human genome.
These dedicated cancer researchers have produced two remarkable discoveries. First, the genetic pattern of cancer is verifiably random. Second, the genetic changes often occur after the onset of cancer, indicating that some cellular changes are occurring well before genes are ever affected. As scientists were investigating both plausible and “improbable” explanations, I asked myself: what is changing that disables the body’s immune system? After critical inquiry and researching scientific sources, I reached the conclusion that the most likely, science-based explanation is that the mitochondria are changing and become impaired.
MITOCHONDRIAL DYSFUNCTION AND CANCER
Scientists point to mitochondrial dysfunction as the genesis of a number of diseases, including cancer. Mitochondrial changes and their relationship to cancer have been proven in a simple experiment. When scientists transfer the nucleus of a cell with cancer genes into a normal cell, the genes revert to normal genes—the cell does not become cancerous. However, when scientists do the reverse, transferring the nucleus of a normal cell to a cancer cell, the cell remains cancerous, suggesting that genes are not driving the cancer. Those engaged in this work have concluded that the driver of cancer is not genetic but dysfunctional mitochondria in cells.
Since food is consumed by mitochondria in the metabolic process, diet is an additional factor involved in mitochondrial dysfunction. Researchers have rediscovered the 1923 conclusions of scientist-physician Otto Warburg, who observed that cancer was caused by a metabolic change—a discovery for which he received a Nobel Prize in 1931. Warburg described how cancer cells stop using oxygen to make energy and shift to non-oxygenated, inefficient metabolism that uses fermentation to produce energy.
Fermentation metabolism causes mitochondria to produce only two ATPs per glucose molecule metabolized, while also forming acidic byproducts. The cell nucleus then changes the gene code to support this impaired metabolism. The fact that the mitochondria change first—before any gene coding changes—undermines the “gene theory” premise of conventional cancer explanations. Most doctors are unaware that the build-up of lactic acid is a result of the cancer, not the originating cause, and so they try to de-acidify the cancer patient’s body by recommending that patients eat an alkaline diet.
MITOCHONDRIAL DYSFUNCTION, DIABETES AND OBESITY
Overconsumption of fructose, the sweetest of the sugars principally found in fruits, is a commonly known cause of diabetes. Whereas table sugar (sucrose) is half glucose and half fructose, high-fructose corn syrup is commonly formulated as 42 percent glucose and 58 percent fructose. Because fructose is a unique sugar metabolized only by the mitochondria in the liver, the culprit that causes diabetes is uric acid, which (as already discussed) is a metabolite of fructose. Though uric acid is a strong antioxidant outside the cell, inside the cell it has the opposite action, producing ROS. In the presence of uric acid, the mitochondria, which should be making the energy-packed ATP, instead produce the energy-depleted ADP.
Once dysfunctional mitochondria stop metabolizing food—and especially thermal-producing fats—the fats accumulate inside the cell and its membrane. The result is twofold: tissues become fat (i.e., fatty liver disease) and fat-laden cell membranes lose permeability, preventing water-soluble sugar from entering the cell. Insulin, which ordinarily allows sugar to enter cells, fails because the accumulated fat prevents sugar’s passage. This condition is called insulin resistance or diabetes. Simultaneously, the liver (fatty liver disease) and body (obesity) begin to store fat. Establishing the etiology of these conditions, a seminal 2013 article in Diabetes stated, “An elevated serum uric acid is. . . one of the best independent predictors of diabetes and commonly precedes the development of both insulin resistance and diabetes. An elevated uric acid also independently predicts the development of fatty liver, obesity, [and] hypertension.”.9 Other researchers have noted that “[f]ewer and smaller-sized mitochondria are found in skeletal muscle of insulin-resistant, obese, or T2DM [type 2 diabetes mellitus] subjects.”10
People who remain thin while eating vast quantities of food often baffle observers. “How can they eat all that food and be so skinny?” A common explanation for what appears to be inexplicable is “high metabolism.” However, scientific understanding of the importance of healthy mitochondria offers a more plausible explanation: overweight people who eat one meal a day but continue to gain weight likely have fewer healthy mitochondria, following injury of the mitochondria by some combination of the factors described earlier. People who remain thin even though they eat a lot likely have high amounts of healthy mitochondria.
MITOCHONDRIAL DYSFUNCTION AND NEUROLOGICAL DISORDERS
Alzheimer’s disease, branded as type 3 diabetes (insulin resistance) by many medical experts, is a condition whereby the “diabetic brain” cannot use sugar and therefore is starved. Magnetic resonance imaging (MRI) of Alzheimer’s patients displays brain shrinkage, an indicator of starvation. Increasingly it has become apparent that feeding and nourishing mitochondria with wholesome ketones—which do not require insulin—can help restore mitochondrial health and relieve brain starvation. Substantiating the potential for some level of recovery, patients with mild cognitive impairment who were fed ketones in the form of medium-chain triglycerides (MCTs)—such as those present in coconut oil—have experienced improvement in brain symptoms.11
Overuse and misuse of antibiotics in childhood also damages mitochondria, which can arrest children’s physical and mental development. Antibiotics are a likely contributor to the neurodevelopmental disorders such as autism and ADHD that are plaguing today’s children.12
FEEDING THE MITOCHONDRIA
For mitochondrial and overall health, individuals must select and eat the highest-quality foods possible—allowing food to be the “medicine” it was meant to be—instead of consuming a diet of denatured processed foods and synthetic vitamins, which insufficiently supports health. The best dietary choices are organic, non-GMO, pasture-raised, grass-fed, traditional foods that are not overprepared, packaged, processed or preserved with additives, colors or chemicals that degrade overall food quality.
Unfortunately, health-conscious individuals often virtuously cite the “grocery list” of high-quality foods that they buy without realizing that they may be failing to prepare these foods properly. Most modern people lack their ancestors’ traditional knowledge and dietary literacy about health-promoting methods of food preparation.
The best place to start and continue on a path to high-quality, properly prepared food is by following the research and accumulated wisdom of the dentist Weston A. Price, a former director of the American Dental Association’s Research Institute. On his scientific travels worldwide, Dr. Price studied the diets of traditional people on five continents. He then assembled the accumulated wisdom of humanity on traditional diets in his seminal treatise, Nutrition and Physical Degeneration, which represents essential reading for anyone concerned with food and health. The Weston A. Price Foundation is a leading advocate for the return to nutrient-dense diets and covers Dr. Price’s findings in its materials and on its website. Nourishing Traditions by Sally Fallon Morell provides comprehensive instruction on proper food preparation techniques.
Mitochondria burn two main fuels: glucose (sugar) and ketones (a normal, carbon-based metabolic product). Because the standard American diet (SAD) supplied by modern food producers has given rise to a widespread sugar addiction, most people tend to burn glucose. Glucose can be considered a “dirty fuel” because its metabolism produces ROS. It is also highly addictive; its removal from the diet can lead to common withdrawal symptoms such as headaches, nausea, malaise and lightheadedness. After becoming more informed about diet and nutrition, replacing a sugar-based diet with a diet based on healthy fats is, therefore, essential. Ketones—made from fat—are the preferred mitochondrial fuel because they are clean-burning, healthy and produce less ROS.
THE ROLE OF AMPK
AMPK (adenosine monophosphate-activated protein kinase) is an enzyme responsible for multiple metabolic functions. High levels of AMPK are found in the liver and brain as well as skeletal muscle. In a complex process, AMPK helps control metabolism by detecting and comparing the quantities of both ADP and ATP. If it senses that low-energy ADP is more abundant than high-energy ATP, AMPK becomes activated. Once activated, AMPK performs a variety of essential metabolic functions:
• It increases the levels of energy-charged ATP
• Changes fat metabolism by lowering triglycerides and raising HDL cholesterol levels, which decreases hard-to-lose visceral fat
• Decreases chronic inflammation
• Initiates autophagy (the purging of cellular trash) and mitophagy (the removal of dysfunctional mitochondria), both of which clean up cellular debris to increase lifespan
• Maintains cellular polarity needed to confirm tissue identity
• Promotes the formation of new mitochondria (mitochondrial biogenesis).
AMPK is not available in foods nor as a supplement but is activated by several herbal plants, some nutrients, lifestyle influences and some prescription medications. Researchers at Spain’s University of Seville also have described how excessive quantities of food can deactivate AMPK and increase oxidative stress, exposing mitochondria to DNA damage.13 These imbalances in AMPK and mitochondrial function can initiate a cascade of inflammatory changes related to metabolic disorders such as obesity and diabetes that are at pandemic levels. As key elements for cellular energy production, AMPK and mitochondria require nutrient and lifestyle changes when imbalanced to activate AMPK as well as treat and remedy metabolic disorders.13
One researcher summarizes how AMPK’s activation of mitochondrial function helps offset diseases and improves overall health as follows:
Indeed, current evidence indicates that AMPK activators may reduce risk for atherosclerosis, heart attack, and stroke; help to prevent ventricular hypertrophy and manage congestive failure; ameliorate metabolic syndrome; reduce risk for type 2 diabetes and aid glycemic control in diabetics; reduce risk for weight gain; decrease risk for a number of common cancers while improving prognosis in cancer therapy; decrease risk for dementia and possibly other neurodegenerative disorders; help to preserve the proper structure of bone and cartilage; and possibly aid in the prevention and control of autoimmunity.14
FURTHER MITOCHONDRIAL CARE
The proper feeding of mitochondria is the most important change to facilitate better health. Lifestyle changes such as intermittent fasting and exercise are also fundamental to wellness. Fasting, or more appropriately, intermittent fasting—a salutary habit of eating only during a six- to ten-hour period each day—increases and resets the mitochondria, changing them from sugar consumers to ketone consumers. During a fast, the body metabolizes body fat until food is available. Some fat is converted to ketones, a preferred fuel, thereby boosting and activating the mitochondria for fat digestion.
Exercise is the second mitochondrial builder. Because exercise requires energy, the mitochondria multiply to supply the extra energy. One researcher explains, “Endurance exercise training increases mitochondria size, number, and oxidative activity.”10 Exercise can explain weight loss through this ability to increase the number of healthy mitochondria. Additional healthy mitochondria not only increase metabolism but also burn more fuel.
HEALTHY AGING
Current scientific research verifies the premise that both declining numbers and dysfunction of mitochondria translate into aging. As a physician-researcher, Dr. Graveline confirms with evidence and personal experience in The Dark Side of Statins that a debilitating aging process results from mitochondrial damage: “The mitochondrial theory of aging proposes that aging, and the development of age-related degenerative diseases, are primarily the result of accumulated oxidative damage to mitochondrial membranes and DNA, over time.”15
Thus supporting mitochondrial health can help control the aging process as well as improve overall health.16 Summarizing the current scientific and medical research, which individuals must consider as fundamental health care knowledge, researchers at the National Institutes of Health have acknowledged that “mitochondria appear to play a central role in regulating cellular life span.”17 Stated another way, cellular longevity enhances human longevity.
Understanding how mitochondrial health relates to overall health equips people to make choices that support wellness, help control body weight and manage the effects of aging—major aspects of happiness and a long life. From these observations about the mitochondria, one can also derive a general principle of cancer prevention: if something is known to cause cancer, it also damages mitochondria, and measures that prevent cancer will likely protect, heal and multiply the number of mitochondria. This knowledge puts the keys to health in each individual’s hands.
An important supplement that the Mitochondria use to produce energy is Coenzyme Q10.
SIDEBARS
LIKELY CONTRIBUTORS OF MITOCHONDRIAL DYSFUNCTION
Many factors—chemicals, foods, modern lifestyle habits and electromagnetic fields—have been implicated as causes of mitochondrial dysfunction. Included in this list are antibiotics, fructose, glyphosate, cell phones and statin drugs, as well as other drugs, chemicals, foods and additives.
ANTIBIOTICS: Many types of radicals are formed in biological systems, but the most worrisome, derived from oxygen, are referred to as reactive oxygen species (ROS) or oxygen free radicals. Dousing mitochondria with antibiotics causes the formation of ROS—a relationship confirmed by scientists writing in 2013 in Science Translational Medicine, who observed that “clinically relevant doses of bactericidal antibiotics. . . cause mitochondrial dysfunction and ROS overproduction in mammalian cells.”3 This is because mitochondrial DNA is similar to the DNA of bacteria, so antibiotics that kill bacteria also harm mitochondria.
FRUCTOSE: An abundance of fructose in the diet, and especially high-fructose corn syrup, overloads mitochondria and halts metabolism. Fructose is then changed to triglycerides, which are the precursor to body fat and eventual obesity. A paper in the Journal of Nutrition and Metabolism explains that uric acid, a “byproduct of uncontrolled fructose metabolism,” increases rapidly following fructose ingestion. In turn, “[u]ncontrolled fructose metabolism leads to postprandial [after eating] hypertriglyceridemia [increased fats in blood], which increases visceral adipose deposition [obesity].”4 In addition, UCLA researchers have described how pancreatic cancer cells readily use fructose to divide and multiply.5
GLYPHOSATE: In 2015, the World Health Organization (WHO) ranked glyphosate as a Class 2A probable carcinogen. Glyphosate originally was patented as an antibiotic and is known to eradicate beneficial, probiotic gut bacteria.6 Again, because the DNA of mitochondria and bacteria are similar, substances like glyphosate that eliminate bacteria may also harm mitochondria.
CELL PHONES: In 2011, the WHO ranked cell phones as a Class 2B possible carcinogen. Studies confirm that changes occur in mitochondrial DNA with exposure to electromagnetic energy transmitted by cell phones. Because Wi-Fi uses the same digital, pulsed signals, it is also implicated in mitochondrial damage. According to researchers at the Centre of Excellence in Biotechnology and Development in New South Wales, Australia, radiofrequency electromagnetic radiation (RF-EMR) “in both the power density and frequency range of mobile phones enhances mitochondrial reactive oxygen species generation.”7
STATIN DRUGS: Duane Graveline, MD, is a retired family physician and former U.S. Air Force flight surgeon. Graveline discusses the effects of cholesterol-lowering statin medications on mitochondria on a webpage titled “PQQ and statin damage.” He says, “Those. . . following my research over the years will know that I consider mitochondrial DNA damage as the ultimate result for many people. . . taking statins.”8
BENEFICIAL FOODS AND SUPPLEMENTS
It is life-enhancing to choose foods and supplements wisely—the more natural the nutrients, the more that the mitochondria respond in beneficial ways. Although mitochondrial supplements are still “a work in progress,” they represent a growth market for “customers looking for an energy boost and an anti-aging solution.”18 The list below summarizes foods and supplements beneficial to mitochondrial health.
HEALTHY FATS: Replacing the low fat/vegetable oil directive that has held sway for forty years, scientific studies as well as current nutritional understanding confirm the dietary wisdom that the healthiest fats are butter; saturated animal fats (such as lard, tallow, chicken schmaltz and duck fat); tropical oils (such as coconut, palm kernel and palm oils); and tree nut fats (such as macadamia). These healthy fats are the foremost recommendation for dietary sources of ketones. Dr. Thomas Seyfried, Yale University and Boston College researcher, argues in Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer in favor of treating cancer nutritionally with saturated fats because they convert to ketones more readily than polyunsaturated oils.19
MCT OIL: “With a couple of exceptions,” state researchers in the Annals of the New York Academy of Sciences, “there is normally no opportunity to consume medium-chain fatty acids from the diet.”.20 Coconut oil and palm kernel oil are the two exceptions. Coconut oil contains C6, C8, C10 and C12 triglycerides. Medium-chain triglyceride (MCT) oil is made from coconut oil but contains only the C8 and C10 triglycerides, which are directly converted in the liver to ketones. For those who do not like the taste or smell of coconut oil, MCT oil has virtually no taste or smell.
PQQ: PQQ (pyrroloquinoline quinone) is the only supplement that increases the number of mitochondria and improves mitochondrial function. It is found naturally in egg yolks (from free-range chickens), vegetables like parsley and celery, and fruits like kiwi and papaya. Integrative physician Isaac Eliaz explains that PQQ is similar to CoQ10 and “is another nutrient that can increase mitochondrial ATP production, while also increasing the number of mitochondria.”21
CO-ENZYME Q10: CoQ10, also known as ubiquinone, is involved in energy production and is abundant in organs like the heart that require high amounts of energy. In fact, CoQ10 is a chemical supplement necessary for mitochondrial survival. With advancing age, when bodily production of CoQ10 declines, an exogenous or dietary supply is required. Organ meats are packed with CoQ10, but most people don’t eat them; as a result, supplementation must provide this valuable nutrient. In The Dark Side of Statins, Dr. Duane Graveline cautions: “Few seniors have the CoQ10 adequacy of their youth. Supplementation is not only important for this group, but critical for most.”15 Others also attest to CoQ10’s importance: “Even as the range of benefits expands beyond mitochondria, CoQ10 remains at the top of mitochondria nutrients.”18
COLOSTRUM: Colostrum—the first milk mothers produce—is not only safe for all babies but is essential for newborns to ingest immediately. Although raw milk is considered nature’s perfect food, colostrum is fifteen times more potent in health-giving properties. Colostrum contains fats, vitamins, minerals, proteins, polypeptides (antibodies), growth factors and antioxidants. In the definitive work on the use of mammalian colostrum, Peptide Immunotherapy: Colostrum, a Physician’s Reference Guide, Dr. Andrew Keech provides biochemical evidence for colostrum as a source of antioxidants. Dr. Keech states, “One such antioxidant, glutathione, has been described as the ultimate antioxidant. It is well-documented that glutathione and its precursors are present in colostrum in relatively high levels.”22 High glutathione levels correlate with long life. In the Journal of Alzheimer’s Disease, researchers contend that colostrum “decelerates the aging process” through “improvement in senescence-associated mitochondrial dysfunction and a decrease in ROS generation.”23
KOMBUCHA: Kombucha is a tart, bubbly drink from the Ural Mountains of Russia. Kombucha is a refreshing source of a potent, detoxifying substance called glucuronic acid. A proven cancer preventive, glucuronic acid works in the liver to convert toxins into harmless forms that the liver can then excrete. A 2011 study published in Pathophysiology found that kombucha tea “modulate[d] the oxidative stress induced apoptosis [i.e., natural programmed cell death] in…hepatocytes probably due to its antioxidant activity and functioning via mitochondria dependent pathways and could be beneficial against liver diseases, where oxidative stress is known to play a crucial role.”24
MAGNESIUM: One of the major mineral deficits of the American diet is magnesium, which functions as a co-factor to vitamin B6, both of which are necessary for the metabolism of proteins. To be biologically active, ATP “must be bound to a magnesium ion,” and “[w]hat is called ATP is often actually Mg-ATP [magnesium-ATP].”25
SELENIUM: Selenium is a nonmetal mineral, which when combined with a protein has antioxidant properties that protect the mitochondrial membrane. Selenium is found in Brazil nuts, fish, red meat, chicken, egg whites and milk. Dietary selenium is required to form biologically active selenoproteins, which are enzymes that function as antioxidants.26 In The Dark Side of Statins, Dr. Graveline confirms that “well over 30 selenoprotein enzymes have been discovered for the element selenium, expressing an unusually wide range of physiological applications with multisystem involvement. These enzymes are highly beneficial in preventing mitochondrial damage, premature aging, and many chronic diseases—similar to the antioxidant role of CoQ1O.”15 However, according to the National Institutes of Health Office of Dietary Supplements website, very high doses of selenium can be toxic.
B VITAMINS: The B vitamins are spark plugs for metabolism—especially B12, B6, thiamin (B1), riboflavin (B2) and niacin (B3). In symbiotic support, B12 is largely responsible for proper formation of every cell in the body, and B6 is necessary for the complete digestion of all proteins. When B2 and B3 are utilized in the body, they convert to FADH2 and NADH, key molecular elements of the ATP-producing Krebs cycle.
D-RIBOSE: D-ribose, which occurs widely in nature, is essential to make the energy-carrying ATP molecule and plays a role in energy recovery and fatigue prevention. D-ribose “comprises the backbone of RNA, the basis of genetic transcription and, through the removal of one hydroxyl group, becomes DNA. Because of this, D-ribose is a promising element of any attempt to repair DNA damage. Additionally, once phosphorylated, ribose can become a subunit of ATP.”27
L-CARNITINE: Found in animal tissue, L-carnitine acts as a carrier, moving fatty acids to the mitochondria. L-carnitine “is our only carrier for fat metabolism and without L-carnitine, all energy potentially derived from fat would be lost.”28
COD LIVER OIL: By providing omega-3 fatty acids, cod liver oil enhances the mitochondrial membrane, which allows the release of ROS, thereby reducing their danger. Research in The Journal of Physiology shows how “the current data strongly emphasize a role of omega-3 in reorganizing the composition of mitochondrial membranes.”29 However, the Weston A. Price Foundation points out that while cod liver oil provides vitamins A, D, and omega-3 fatty acids, it’s important to balance these with omega-6 arachidonic acid from animal fats.
ALPHA-LIPOIC ACID: Alpha-lipoic acid (ALA) is present in meat as well as vegetables and fruits in smaller quantities. ALA provides mitochondrial antioxidant effects and delays body aging. In an article in Metabolism, researchers affirm the disease-preventive qualities of lipoic acid, which “possesses antioxidative and antidiabetic properties.”30
REFERENCES
1. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951-7.
2. Munnich A, Rötig A, Chretien D et al. Clinical presentation of mitochondrial disorders in childhood. J Inherit Metab Dis. 1996;19(4):521-7.
3. Kalghatgi S, Spina CS, Costello JC et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci Transl Med. 2013;5(192):192ra85.
4. Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab. 2013;2013:682673.
5. Liu H, Huang D, McArthur DL et al. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010;70(15):6368-76.
6. Samsel A, Seneff S. Glyphosate, pathways to modern diseases II: celiac sprue and gluten intolerance. Interdiscip Toxicol. 2013;6(4):159-84.
7. De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009;4(7):e6446.
8. Graveline D. PQQ and statin damage. Spacedoc, Feb. 2016. https://spacedoc.com/articles/pqq-and-statin-damage.
9. Johnson RJ, Nakagawa T, Sanchez-Lozada LG et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307-15.
10. Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401-14.
11. Fortier M, Castellano CA, Croteau E et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15(5):625-34.
12. Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23.
13. Bullon P, Marin-Aguilar F, Roman-Malo L. AMPK/mitochondria in metabolic diseases. Exp Suppl. 2016;107:129-52.
14. McCarty MF. AMPK activation—protean potential for boosting healthspan. Age (Dordr). 2014;36(2):641-63.
15. Graveline D. The Dark Side of Statins. Spacedoc Media; 2017, p. 129.
16. Feister W. How to Care & Feed Your Mitochondria: Personal Healthcare Primer. Pura Vida Media; 2018.
17. Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun. 2002;294(2):245-8.
18. CoQ10 leads the mitochondrial supplements category. https://www.newhope.com/vitamins-and-supplements/coq10-leads-mitochondrial-supplementscategory.
19. The benefits of a ketogenic diet and its role in cancer treatment. https://articles.mercola.com/sites/articles/archive/2013/06/16/ketogenic-diet-benefits.aspx.
20. Cunnane SC, Courchesne-Loyer A, St-Pierre V et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci. 2016;1367(1):12-20.
21. Eliaz I. Telomeres, mitochondria, and new age of aging. Holistic Primary Care. 2017;18(1):8.
22. Keech AM. Peptide Immunotherapy: Colostrum, a Physician’s Reference Guide. China: AKS Publishing; 2009, p. 242.
23. Boldogh I, Kruzel ML. Colostrinin: An oxidative stress modulator for prevention and treatment of age-related disorders. J Alzheimer’s Dis. 2008;13(3):303-21.
24. Bhattacharya S, Gachhui R, Sil PC. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology. 2011;18(3):221-34.
25. Magnesium in biology. https://en.wikipedia.org/wiki/Magnesium_in_biology.
26. Selenium. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/.
27. D-ribose. https://spacedoc.com/articles/d-ribose.
28. Why L-carnitine? https://spacedoc.com/articles/07-why-l-carnitine.
29. Herbst EA, Paglialunga S, Gerling C et al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol. 2014;592(6):1341-52.
30. Wang Y, Li X, Guo Y, Chan L, Guan X. Alpha-lipoic acid increases energy expenditure by enhancing AMPK-PGC-1α signalling in the skeletal muscle of aged mice. Metabolism. 2010;59(7):967-76.
This article appeared in Wise Traditions in Food, Farming and the Healing Arts, the quarterly journal of the Weston A. Price Foundation, Fall 2019






